In this article, we will examine the properties of free radical using the octet rule. The octet rule can be easily understood as an example of hydrogen atom, helium atom, oxygen atom.
What is the octet rule?
Understand the bizarre behavior of free radicals that destroy healthy muscle fibers (muscular tendon ligaments, shoulder rotator cuff) and brain cells (:))? To do that, you need to know a chemical theory called the octet rule. There are many people who are unfamiliar, but it's not really difficult to hear the explanation~:)
The octet rule is a chemical theory that states that each atom constituting a molecule is in the most stable state when 8 electrons enter the outermost shell. (2 in the first shell) Every atom has an atomic nucleus in the center and an electron outside. And the atomic number is assigned according to the number of electrons.
The octet rule is that the atom is in a stable state when the electron follows the following rule. In other words, the first shell is 2, the next shell is 8, and the next shell is also 8… Only in this way is it in a stable state.
Case of hydrogen atom
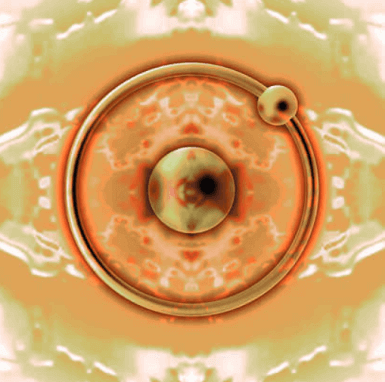
For example, a hydrogen atom with atomic number 1 has 1 electron. This is not stable according to the octet rules. And it needs 1 electron to stabilize. So, it stabilizes itself by taking one electron from the surrounding atoms.
<Octet rule and pure hydrogen atom (has 2 electrons)>
In this case, the atomic nucleus of hydrogen has a charge of +1, but since there are two electrons, the charge of -2 becomes +1 – 2 = -1. (The nucleus has a charge of +1, and the electron has a charge of -1) So -1 becomes a hydrogen anion. This -1 hydrogen anion is stable because it has two electrons according to the octet rule, so it can exist as it is in nature. (Look at ‘the first 2’ in the picture above)
Case of helium atom
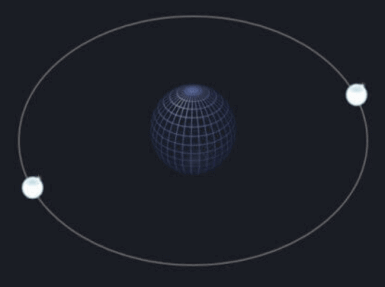
Next, helium, atomic number 2, has two electrons. Therefore, since it follows the octet rule, it can exist in nature stably as a pure atom. (Look at ‘the first 2’ in the picture above)
Case of oxygen atom
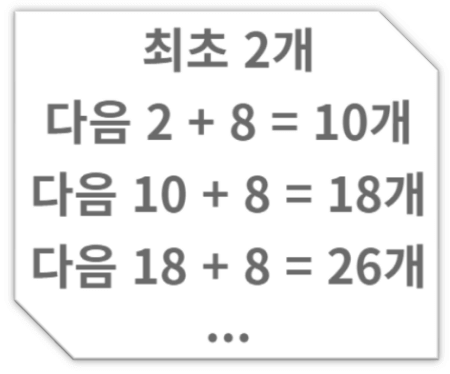
Next, the atomic number of oxygen is 8. According to the octet rule, 6 electrons must be discarded to become 2 electrons or 2 electrons must be obtained to make 10 electrons to stabilize.
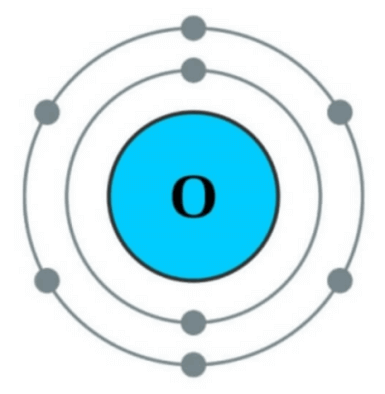
In this case, it is easier to get 2 electrons than to discard 6 electrons. Therefore, the pure oxygen atom with the number of electrons of 8 becomes bloody trying to steal the two insufficient electrons from the surrounding atoms. These pure oxygen atoms themselves are called free radical because they are highly reactive.
In this case, the atomic nucleus of oxygen has a charge of +8, but since there are 10 electrons, the charge of -10 becomes +8 – 10 = -2. (The nucleus has a charge of +1, and the electron has a charge of -1) So -2 becomes an oxygen anion. This -divalent oxygen anion is stable because it has 10 electrons according to the octet rule, so it can exist as it is in nature. (Notice ‘Next 2 + 8 = 10 pieces’ in the picture above)
Epilogue
Above, we have examined the properties of free radical using the octet rule. To exist in nature, it must be in a stable state. Pure hydrogen atom and pure oxygen atom are not in a stable state according to the octet rule, so they take electrons from the surroundings. So it becomes a hydrogen anion and an oxygen anion. On the other hand, pure helium atoms are in a stable state according to the octet rule, so there is no need to take electrons from the surroundings. In conclusion, it can be seen that the hydrogen anion, oxygen anion, pure helium atom that exists in a stable state in nature.
Hydrogen atom and hydrogen anion, oxygen atom and oxygen anion. Only the number of electrons was different, but they were the same! In the next article, we will look at these negative ions.
Thank you for subscribing so far. I was Mondolly~ :)


댓글 남기기